Tools & Technology
Tools & Technology
The program develops and applies state-of-the-art optical, molecular, and electrophysiological technologies to study the neural mechanisms of learning and memory. Our approach integrates in vivo and in vitro imaging, all-optical circuit interrogation, genetic manipulation, and transcriptomic profiling to bridge processes that span from single synapses to large neuronal populations.
High-Speed 3D Imaging of Neural Circuits – Femtonics FEMTO3D Atlas
Applications: subcellular voltage and calcium imaging, spine plasticity, and fast dendritic recordings during behavior.
The FEMTO3D Atlas is our flagship two-photon imaging platform for in vivo subcellular and population-scale recordings. Its acousto-optic deflector (AOD) scanning allows rapid, random-access imaging at up to 100 kHz, capturing neuronal and synaptic activity across large 3D volumes with motion-correction precision.
Key Capabilities:
Acousto-Optic Deflector (AOD) random-access scanning at up to 100 kHz
Real-time 3D motion correction for imaging in awake, behaving animals
Dual-laser configuration for simultaneous optogenetic stimulation and imaging
Sub-micron spatial and sub-millisecond temporal resolution for recording synaptic plasticity in vivo
This system enables subcellular voltage and calcium imaging from dendrites and spines during behavior, and functional studies of synaptic plasticity and network dynamics at single-cell resolution. The Femtonics platform is a cornerstone for our efforts to reveal how synaptic and dendritic events encode experience and contribute to stable memory traces.
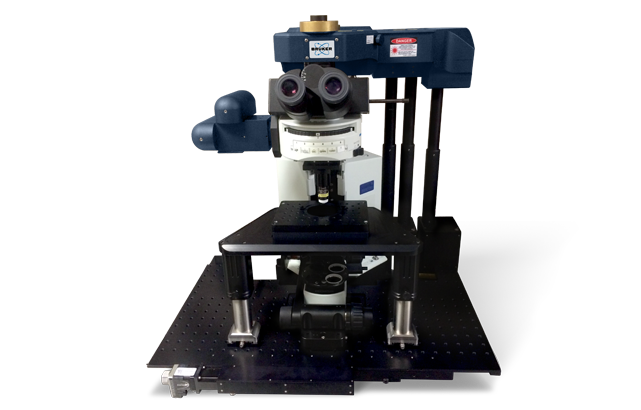
All-Optical Imaging and Physiology – Bruker Ultima 2Pplus with NeuraLight 3D Ultra SLM
Applications: all-optical physiology, circuit mapping, and deep-layer voltage imaging.
The Bruker Ultima 2Pplus coupled with the NeuraLight 3D Ultra Spatial Light Modulator (SLM) provides an advanced system for simultaneous multiphoton imaging and holographic photostimulation.
Key Capabilities:
Modular two- and three-photon imaging with a field of view up to 28 mm FN
High-sensitivity GaAsP detection enabling imaging up to 1.3 mm depth and three-photon capability for deeper layers
3D holographic photostimulation of neurons or synapses using Spatial Light Modulation (SLM) with subcellular precision across multiple focal planes
Fast pattern switching (≤ 1.6 ms) for dynamic activation of neuronal ensembles
Full synchronization with electrophysiology, voltage imaging, and behavioral control systems
This platform allows precise activation or silencing of functionally defined cells during ongoing imaging, supporting causal experiments in neural coding and circuit computation.
In Vitro Circuit Physiology – Femtonics Atlas 3D Dual-Laser System
Applications: in vitro slice physiology, connectivity mapping, and synaptic plasticity studies using human and murine brain tissue.
For detailed circuit analysis, the lab operates a dedicated Femtonics Atlas 3D dual-laser microscope optimized for in vitro slice physiology.
Key Capabilities:
Dual-laser inputs for concurrent two-photon imaging and glutamate uncaging
Optimized for multi-patch electrophysiology and in vitro two-photon imaging
Multiple Luigs & Neumann micromanipulators for multi-patch recordings in human and mouse brain slices
Sub-micron targeting precision to investigate synaptic connectivity and plasticity rules within microcircuits
Enables mapping of synaptic connectivity between identified neurons in acute human and mouse slices
This setup supports experiments mapping connectivity among identified neurons and probing how specific synaptic interactions contribute to circuit stability and learning. It supports in vitro plasticity experiments that combine optical stimulation, electrophysiology, and pharmacological manipulation. This platform bridges detailed circuit physiology with optical imaging and genetic perturbation, serving as a cornerstone for mechanistic studies of human and rodent hippocampal networks.
Targeted Gene and Protein Manipulation – Bruker Ultima Electroporation System
Applications: targeted genetic and protein manipulation in individual neurons.
Our Bruker Ultima microscope is additionally equipped with in vivo single-cell electroporation instrumentation.
Key Capabilities:
Integrated with the Ultima multiphoton imaging system for visual guidance during electroporation
Fine micropipettes deliver brief electrical pulses to transiently permeabilize membranes of visually identified neurons
Allows delivery of plasmid DNA, mRNA, or CRISPR constructs into individual cells
Enables cell-specific expression of fluorescent reporters, optogenetic tools, or genetically encoded sensors
This capability provides causal control over neuronal gene and protein expression, linking molecular mechanisms to circuit physiology. Supports experiments investigating cell-autonomous signaling, synaptic integration, and gene function within intact neural circuits.
Freely Moving Population Imaging – Thorlabs Mini2P
Applications: population calcium imaging during naturalistic behaviors and across brain states.
The Thorlabs Mini2P miniature two-photon microscope enables cellular-resolution calcium imaging in freely moving animals.
Key Capabilities:
Lightweight head-mounted design (≈ 5 g) with high-speed MEMS scanning
Fast multiplane acquisition via micro-tunable lens (< 0.4 ms response)
Monitors hundreds of neurons across diverse brain states—wake, exploration, and sleep
Compatible with prisms and GRIN lenses for deep-structure access (e.g., hippocampus)
Recordings from hundreds of neurons with optional large-FOV or high-speed configurations
By coupling Mini2P imaging with behavioral and virtual-reality paradigms, we investigate how distributed ensembles encode and consolidate memory.
Correlative Transcriptomics – MERSCOPE Ultra Spatial Genomics Platform
Applications: correlative mapping of gene expression with functional imaging data.
To connect function with molecular identity, the lab employs MERSCOPE Ultra, a high-plex MERFISH 2.0-based spatial transcriptomics system.
Key Capabilities:
MERFISH 2.0 sensitivity for single-cell transcript detection in mouse or human tissue
Maps up to 1,000 genes at single-cell resolution over 3 cm² tissue areas
High-resolution mapping of RNA species across cell types and layers
Preserves tissue architecture, enabling direct registration with functionally imaged neurons
Enables correlative in vivo imaging → molecular profiling workflows
This approach reveals gene expression programs underlying synaptic stability, aging, and Alzheimer's-related vulnerability. This approach closes the loop between optical physiology and gene expression, creating comprehensive datasets that couple activity, structure, and molecular state.
Complementary Laboratory Systems
Patch-Clamp and Electrophysiology Rigs: for in vitro and ex vivo recordings in mouse and human tissue, synchronized with optical imaging
Optical Stimulation Modules (LED and Laser): integrated for full-field or targeted activation
Computational and Data Infrastructure: dedicated GPU clusters for online voltage-signal analysis, imaging data reconstruction, and modeling of network dynamics
Behavioral Platforms: virtual-reality and freely moving setups synchronized with imaging and stimulation systems
Integrated Experimental Framework
Together, these technologies provide a unified experimental platform to observe, manipulate, and decode neural mechanisms of memory:
Subcellular imaging of synaptic and dendritic plasticity during learning
High-speed voltage imaging of dendritic and somatic compartments across brain states
All-optical interrogation and readout of circuit function
Genetic and molecular manipulation of individual neurons
Targeted manipulation of protein and gene expression in individual neurons
In vitro plasticity and connectivity mapping in human and murine brain tissue
Population imaging in freely behaving animals using Mini2P
Correlative transcriptomic profiling of identified memory circuits